Our epidemic intelligence team regularly publishes COVID Focus Reports on the global state of vaccination efforts. Here’s a sampling of what’s in the most recent report.
April 8, 2021
Global administration
- As of April 7, five countries/territories have vaccinated more than 50% of their population, including Gibraltar (96%), Falkland Islands (83%), Seychelles (66%), Bhutan (62%), and Israel (59%).
- On March 27, Bhutan began its vaccination campaign, aiming to become the first country to administer first doses to its entire eligible adult population within a single week. Bhutan has shown a world-leading response to the pandemic overall, with 35 cases and 0 deaths as of April 7 in a population of roughly 700,000.
Spotlight on Stable Re-openings
- As Israel has one of the world’s highest rates of vaccination coverage, it serves as a real-world example of a country’s ability to re-open its domestic economy in a stable manner while its COVID-19 epidemiological situation improves.
- BlueDot will soon release projected dates of stable re-openings worldwide. As part of that work, we sought to identify a reasonable, real-world benchmark of a country’s population-level immunity to COVID-19.
- With Israel’s experience thus far as a first real-world example, we estimate that one of the benchmarks for stable re-opening is when at least 60% of a country’s population acquires immunity to COVID-19. This benchmark may move occasionally as time elapses and data emerges from more countries.
- To derive our benchmark, we rounded the estimated 61.0% of Israel’s population with immunity as of March 7, 2021 where a subsequent surge in hospitalizations, deaths, and cases amidst re-opening was not observed. Thus, we estimate that a benchmark of at least 60% of a country’s total population having acquired immunity may be sufficient to begin a safe re-opening of its economy in a gradual data-driven fashion.
- The United Kingdom will be another real-world example as they are nearing this benchmark and beginning gradual re-openings. Early experience from the U.S. and Chile suggests the COVID-19 epidemiological situation may worsen if re-openings occur prior to this benchmark being achieved.
Vaccine research
- There are 23 COVID-19 vaccine candidates currently being studied in Phase 3 or Phase 2/3 trials. No new vaccine candidates have entered Phase 2/3 or Phase 3 trials since our previous report on March 24.
BlueDot’s best-in-class epidemic intelligence — which includes regular reports like the one excerpted here — has helped businesses and governments around the world throughout the COVID-19 pandemic.
March 26, 2021
What are COVID-19 vaccine passports?
- As vaccination campaigns continue, many governments are looking for ways to lift existing restrictive physical distancing measures imposed to control the pandemic. The idea of a COVID-19 vaccine passport or certificate is a response to global lockdown fatigue and the desire of citizens, corporations, and governments to reopen their economies.
- COVID-19 vaccine passports are intended to be used as a form of certification of COVID-19 vaccination that can be stored digitally or on paper and used for international travel and border control. This would be a key strategy to control the spread of SARS CoV-2 and its variants. Such a passport could impose an artificial restriction participation in social and economic activities.
Spotlight on Vaccine Passports
What are COVID-19 vaccine passports?
- As vaccination campaigns continue, many governments are looking for ways to lift existing restrictive physical distancing measures imposed to control the pandemic. The idea of a COVID-19 vaccine passport or certificate is a response to global lockdown fatigue and the desire of citizens, corporations, and governments to reopen their economies.
- COVID-19 vaccine passports are intended to be used as a form of certification of COVID-19 vaccination that can be stored digitally or on paper and used for international travel and border control. This would be a key strategy to control the spread of SARS CoV-2 and its variants. Such a passport could impose an artificial restriction on participation in social and economic activities.
Which countries are considering using vaccine passports?
- Asia: China, Russia, India, Japan, Thailand, Israel, Bahrain, Saudi Arabia, United Arab Emirates
- Africa: African Union countries
- North America: United States, Canada
- South America: Chile
- Europe: European Union countries, United Kingdom
- Oceania: Australia, New Zealand
How do COVID-19 vaccine passports differ from existing vaccine certificates?
- The idea of vaccine passports or certificates is not new. During the smallpox vaccination campaigns, the smallpox vaccine scar served as a proof of vaccination to gain access to travel. In many countries, proof of immunization for polio, measles, rubella, and mumps are required for school entry. International health regulations currently require certification for Yellow Fever vaccination if arriving from an affected area as designated by the World Health Organization. That said, COVID-19 vaccine passports are the first global effort to digitize vaccination records and to use digital immunization passports to safely open activities to those who prove their immunity.
Which parties are developing them?
- The International Air Transport Association (IATA), which represents nearly 300 airlines that collectively carry 82% of all air travelers globally, have been urging governments to create systems to speed the return of the international travel industry since vaccinations became available.
- In March, IATA announced its plan to introduce a mobile phone application to enable arriving travellers to prove they’ve been vaccinated or tested negative for COVID-19. The application, named The Travel Pass, could become necessary for both international and domestic flights should airlines begin requiring all their passengers to document their COVID-19 vaccination and testing status.
- There are other non-profit and private-sector vaccine passport initiatives underway. IBM has developed an application called the Digital Health Pass and has partnered with Moderna to track its vaccination administration for travellers. IBM has also begun a pilot program with New York State in the U.S. to test the Digital Health Pass as a way to maintain public safety in spaces where people congregate.
- As well, the World Health Organization has been partnering with Estonia on a pilot COVID-19 vaccine certificate project with potential for global standardization and adoption. Furthermore, the Vaccination Credential Initiative (VCI), which consists of more than 200 partners including Microsoft, Oracle, and many other technology companies and healthcare providers, was formed in January with a vision to set software standards for security of private data.
- To date globally, there exist many decentralized and parallel approaches across organizations to develop COVID-19 vaccine passports. As international bodies, national governments, nonprofit organizations, and private sector groups work to negotiate consistent global standards, the challenges of implementing a universally recognized vaccine passport system remain substantial.
What are the pros and cons of vaccine passports?
- Advocates of COVID-19 vaccine passports have promoted the idea of proof of vaccination as a way to accelerate post-pandemic international travel and boost the recovery of the global economy.
- However, there are concerns about privacy and discrimination. Ethics experts have argued that requiring proof of vaccination is “an unacceptable violation of civil liberties”, while adding that such requirements will “create inequitable outcomes globally” and governments must make exceptions for individuals who cannot be vaccinated due to pre-existing conditions or claims of religion or conscience.
- Clear coherent rules are needed across the public and private sectors to ensure good stewardship of vaccine certification requirements as well as a centralized database that ensures privacy to assist secure sharing of health information.
- Scientifically, experts have raised concerns that although available vaccines demonstrate effectiveness in preventing serious illness, the possibility that vaccinated travelers could still contribute to the transmission of the virus does exist and that ultimately, on-ground testing may be still needed.
Administration
- Israel has begun to face vaccine hesitancy among Arab Israelis and Israeli Haredi Jews. Overall vaccination rates in the country have substantially decreased.
- According to a study published in the New England Journal of Medicine, antibody levels among individuals with pre-existing immunity due to prior infection were 10 to 45 times higher than those without preexisting immunity at the same time points after the first vaccine dose.
Research
- There are 23 COVID-19 vaccine candidates currently being studied in Phase 3 or Phase 2/3 trials. A vaccine candidate developed by the Reithera/Lazarro Spallanzani National Institute for Infectious Disease in Italy has entered Phase 2/3 trials and a candidate developed by the Center for Genetic Engineering and Biotechnology of Cuba has entered Phase 3 trials since our previous report on March 10.
BlueDot’s best-in-class epidemic intelligence — which includes regular reports like the one excerpted here — has helped businesses and governments around the world throughout the COVID-19 pandemic.
March 11, 2021
Spotlight on Intranasal Vaccines
Current intramuscular COVID-19 vaccines in use are able to reduce symptomatic cases and prevent severe illness but may still allow for asymptomatic infection and the potential to transmit the virus to other individuals. This is because the virus can live in the mucosa of the nose and throat where the immune system is not able to reach through intramuscular vaccination. Animal studies suggest that live SARS-CoV-2 virus can remain in the nasal cavity even after leaving the lungs, thereby leaving the possibility of transmission after intramuscular vaccine administration.
As a result, researchers have proposed intranasal vaccines that are designed to trigger immune defenses in the mucosa when inhaled directly into the nose. Other benefits of intranasal vaccinations compared to current intramuscular vaccines include:
- They are relatively painless. The absence of a needle may also encourage those who decline injections to consider the vaccine.
- May be self-administered at home with minimal instruction.
- May require no refrigeration, allowing for easy transport and storage, especially in low-income countries.
- Easier to mass produce and distribute to populations.
- Efficient distribution, especially if periodic booster vaccinations are needed.
However, of the hundreds of vaccine candidates that have been approved and currently in various stages of development globally, only a small number are designed for intranasal delivery. A few of the early research and development efforts focused on the mucous route in animal studies have reported promising results.
As of March 10, four intranasal COVID-19 vaccine candidates, developed in China, India, U.K., and the U.S., have progressed to Phase 1 trials.
Administration
- As of March 10, there are four countries/territories that have vaccinated more than 50% of their populations:
- Gibraltar (84%),
- Falkland Islands (65%),
- Seychelles (59%),
- and Israel (56%)…
- Israel reopened hotels and gyms on February 23 and restaurants for indoor dining on March 1 for those who are fully vaccinated or deemed immune after recovering from COVID-19. The country is also planning to restart international tourism soon as part of its gradual return to normal business activities…
- A growing number of countries are considering “COVID-19 vaccine passports”. On March 1, the European Commission released its proposed “Digital Green Pass,” designed to permit European citizens to safely travel across the European Union or abroad..
Variants of Concern
- No significant difference of vaccine effectiveness against the B.1.1.7 variant (initially detected in the U.K.) compared to the existing predominant strain was observed in Pfizer/BioNTech, Moderna, AstraZeneca, and Novavax’s vaccines.
- Reduction in effectiveness was observed in Moderna, AstraZeneca, and Johnson and Johnson’s vaccine against the B.1.351 variant (initially detected in South Africa).
- Pfizer/BioNTech and Moderna are both testing a third booster dose to evaluate efficacy against known variants of concern.
Research
- There are 21 COVID-19 vaccine candidates currently being studied in Phase 3 or Phase 2/3 trials. One new vaccine candidate developed by the Research Institute for Biological Safety Problems (RIBSP) in Kazakhstan has entered Phase 3 trials since our previous report on February 24.
BlueDot’s best-in-class epidemic intelligence — which includes regular reports like the one excerpted here — has helped businesses and governments around the world throughout the COVID-19 pandemic.
February 11, 2021
Administration
- As of February 10, Israel’s vaccine administration rate continues to lead all other countries, with more than 40% of its population having received at least one dose of either the two-dose Pfizer/BioNTech or Moderna vaccine. The average vaccination rate in Israel is approximately 110,000 people per day.
- Between January 15 and February 6, 2021, Israel demonstrated a reduced number of daily new cases and hospitalizations among those who received vaccination.
- A four-fold decrease in viral load in specimens collected from vaccinated COVID-19 patients was identified 12 or more days after vaccination, corresponding to the expected onset of early vaccine protection. This suggests that the Pfizer/BioNTech vaccine can potentially contribute to reduced transmissibility.
- However, Israel is now reporting a sharp rise in the number of COVID-19 cases among children and adolescents.
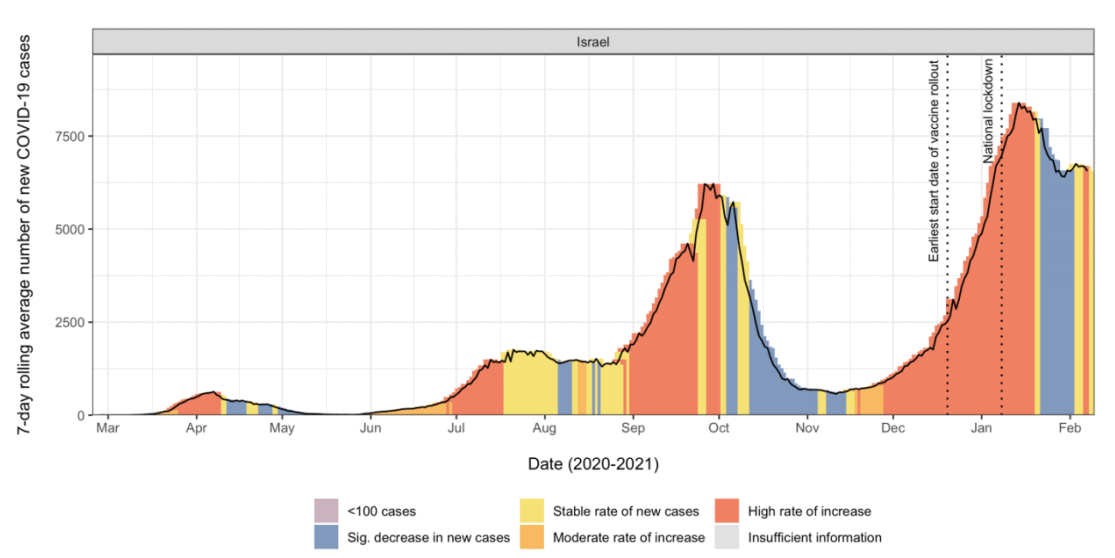
Variants of concern
- Most studies available as of February 10, 2021, report no significant difference of vaccine effectiveness against the B.1.1.7 variant (initially detected in the U.K.) compared to the existing predominant strain. However, reduction in effectiveness was observed in Moderna, AstraZeneca, and Johnson and Johnson’s vaccine against the B.1.135 variant (initially detected in South Africa). Plans are underway to address the reduced effectiveness against the variants of concern.
Research
- 20 COVID-19 vaccine candidates are being studied in Phase 3 or Phase 2/3 trials. No new vaccine candidates have entered Phase 3 or Phase 2/3 trials since our previous report from January 27..
BlueDot’s best-in-class epidemic intelligence — which includes regular reports like the one excerpted here — has helped businesses and governments around the world throughout the COVID-19 pandemic.
January 27, 2021
Administration
- As of January 27, Israel’s vaccine administration rate continues to lead all other countries, with more than 31% of its population having received at least one dose of either the two-dose Pfizer/BioNTech or Moderna vaccine. The average vaccination rate in Israel is approximately 110,000 people per day.
- Gibraltar, an overseas territory with a relatively small population of 33,000, also has 31% of its population initiating vaccines as of January 26. Gibraltar has been vaccinating its population since January 9 with Pfizer/BioNTech’s vaccine at a rate of approximately 585 people per day on average.
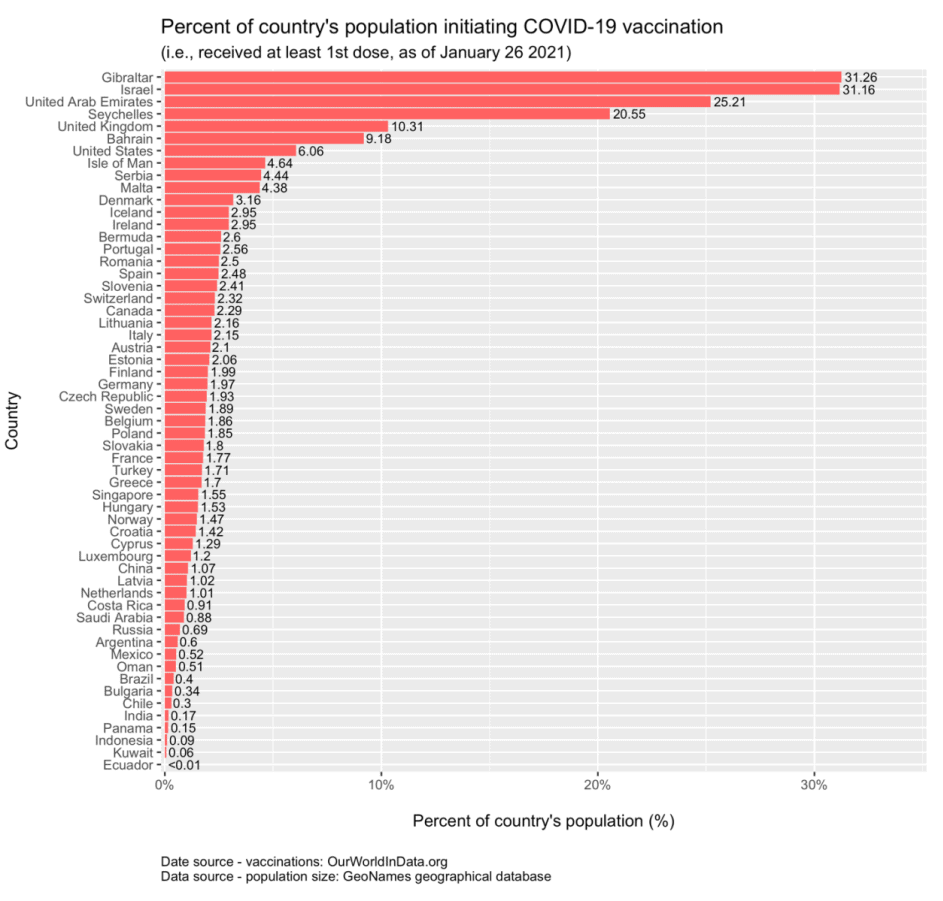
Adverse Reactions
- Anaphylaxis remains one of the most frequently observed serious adverse reactions among those who have received Pfizer/BioNTech’s vaccine. According to data collected from December 14 to 23, 2020, approximately 11.1 cases of anaphylaxis cases were reported per 1,000,000 doses administered.
- 23 deaths were reported among the elderly within a few days of receiving Pfizer/BioNTech’s vaccine in Norway. However, the WHO’s COVID-19 Vaccine Safety subcommittee concluded the vaccine was not significant in contributing to these deaths and that vaccination remains favourable in the frail elderly.
Variants
- Small preprint studies suggest Pfizer/BioNTech’s and Moderna’s vaccines are as effective in producing levels of antibodies against variant B.1.17 (initially detected in the U.K.) as against existing predominant strains.
- However, for Moderna’s vaccine, a six-fold reduction in levels of antibodies were observed with the B.1.351 variant (initially detected in South Africa) relative to the existing dominant strains. Moderna is planning to test a third booster dose of its vaccine to study the ability to increase the levels of antibodies its vaccine produces against emerging variants beyond the existing dominant SARS-CoV-2 strains.
- Moderna is also developing another emerging variant booster candidate (mRNA-1273.351) against the B.1.351 variant with plans to test it in a new Phase 1 clinical trial soon.
Research
- 20 COVID-19 vaccine candidates are being studied in Phase 3 or Phase 2/3 trials. No new vaccine candidates have entered Phase 3 or Phase 2/3 trials since our previous report.