A phase 2 open-label trial is now underway in Rwanda as the country seeks to contain further local spread
This past weekend, the Washington, DC-based Sabin Vaccine Institute delivered an additional 1,000 doses of a vaccine in development to the Rwandan capital of Kigali, amid the country’s first-ever recorded outbreak of Marburg virus. The outbreak is centered around the King Faisal and University Teaching hospitals in Kigali, with 65 identified cases and 15 deaths reported to date. Known for causing haemorrhagic fever, Marburg’s case fatality rates in previous outbreaks have reached as high as 90%.
This latest vaccine shipment comes on the heels of an initial shipment of 700 doses one week earlier. By October 16, 771 of those doses had already been administered, as Rwandan and African public health authorities work to contain further spread of the current outbreak. More than 3,700 tests have been performed to date, and at least 36 close contacts of confirmed cases have been isolated. Official reports indicate that, thus far, all cases have been confirmed within a cluster at a Kigali hospital and their close contacts, suggesting there is no community transmission.
While Rwandan authorities have been successful to date in tracking the virus and administering vaccine doses, this outbreak is part of a broader, disconcerting trend with Marburg, says BlueDot head of surveillance Dr. Mariana Torres Portillo. “Marburg virus outbreaks used to happen every five years in the past,” Dr. Portillo says. “This year’s outbreak marks the third consecutive year with an outbreak, which only underlines the importance of the work that authorities are doing and of the vaccine and treatment trials now underway in Rwanda.”
Marburg: discovery, history, presentation
The Marburg virus is a member of the Filoviridae family of viruses, the best known of which is the Ebola virus, though Marburg virus was discovered nearly a full decade before Ebola, in 1967. That year, infected African green monkeys were transported for medical testing from Uganda to the cities of Belgrade (then part of Yugoslavia), Frankfurt and Marburg, Germany, where the first known outbreak occurred, thus giving the virus its name.
Since then, there have been only nine known outbreaks of Marburg virus across the African continent, the most prominent being the 2004-05 outbreak in Angola, where the virus infected 252 people and killed 227. In some smaller outbreaks, all infected individuals have died of the disease; in other outbreaks, case fatality rates have ranged from 33% to 75%. Perhaps most noteworthy is the increasing frequency and distribution of Marburg virus outbreaks, which have been recorded in four different countries in the last 3 years: Guinea, Ghana, Equatorial Guinea and Tanzania.
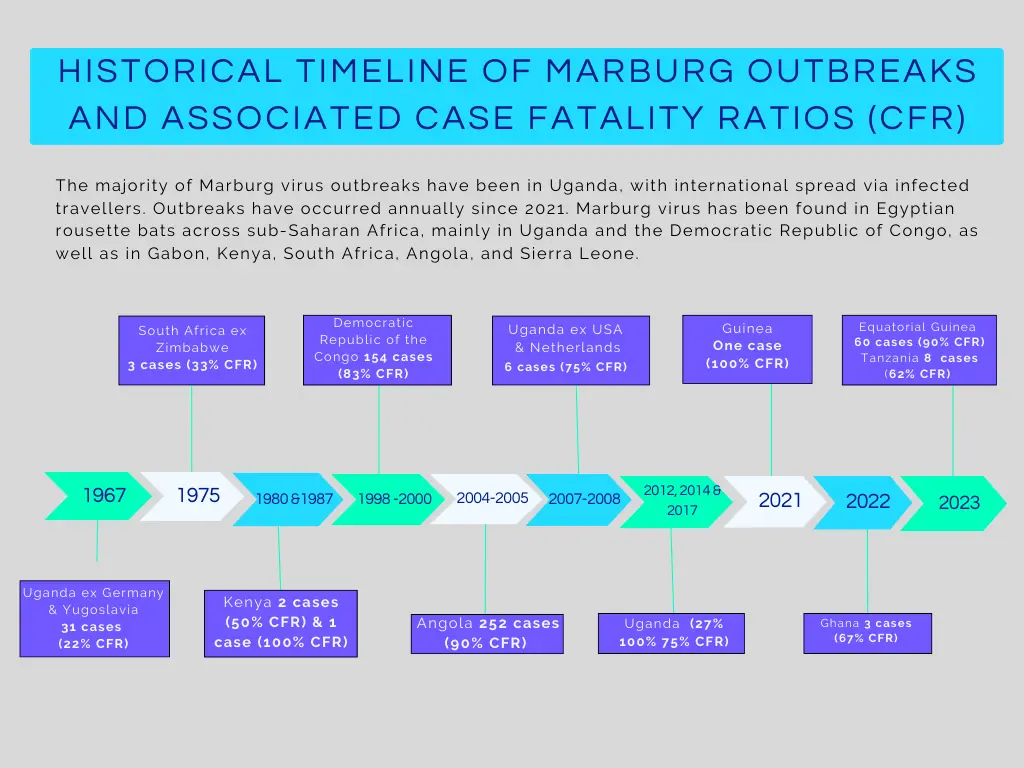
Source: BlueDot and https://www.cdc.gov/marburg/outbreaks/index.html
Similar to other infectious disease trends, this increase is likely multifactorial including improved testing, increased urbanization, population movement, and climate change. Moreover, it’s possible that some Marburg virus outbreaks go undetected. More research is needed to determine if the distribution of the virus’ natural hosts, fruit bats, is changing, and if they are coming into more frequent contact with humans.
Marburg is transmitted via direct contact with blood and other body fluids of infected people and animals — most notably fruit bats — or by indirect contact with contaminated surfaces such as clothing, bedding and medical equipment. Though rare, the disease it causes is severe.
The virus’ incubation period typically lasts from 5 to 10 days (with a range of up to 21 days), during which infected individuals are not considered contagious. The onset of symptoms is abrupt, with a fever reaching as high as 40°C, as well as the rapid development of headache, myalgia, diarrhea and vomiting. Within a week the disease can progress to mucosal, gastrointestinal and venipunctural bleeding, followed by disorientation, seizures and coma. In some survivors, the disease never reaches the haemorrhagic phase.
Notably, 80% of cases in the current Rwandan outbreak are health care workers. This can happen when spread occurs before the outbreak is determined to be due to Marburg virus, and hence appropriate PPE protocols are not yet in place. This speaks to the need for better protection for those most at risk in endemic regions, underlining the value of the vaccine trials now underway.
Marburg in Rwanda: Risk of ground and air travel spread
Given Rwanda’s quick and effective response, the Africa Centres for Disease Control (Africa CDC) recently stated it expects to see the end of the outbreak soon. While Marburg’s spread has slowed, there are still new cases being identified. And given the interconnectedness of the Kigali region to neighbouring provinces and countries, there is still some risk of broader spread.
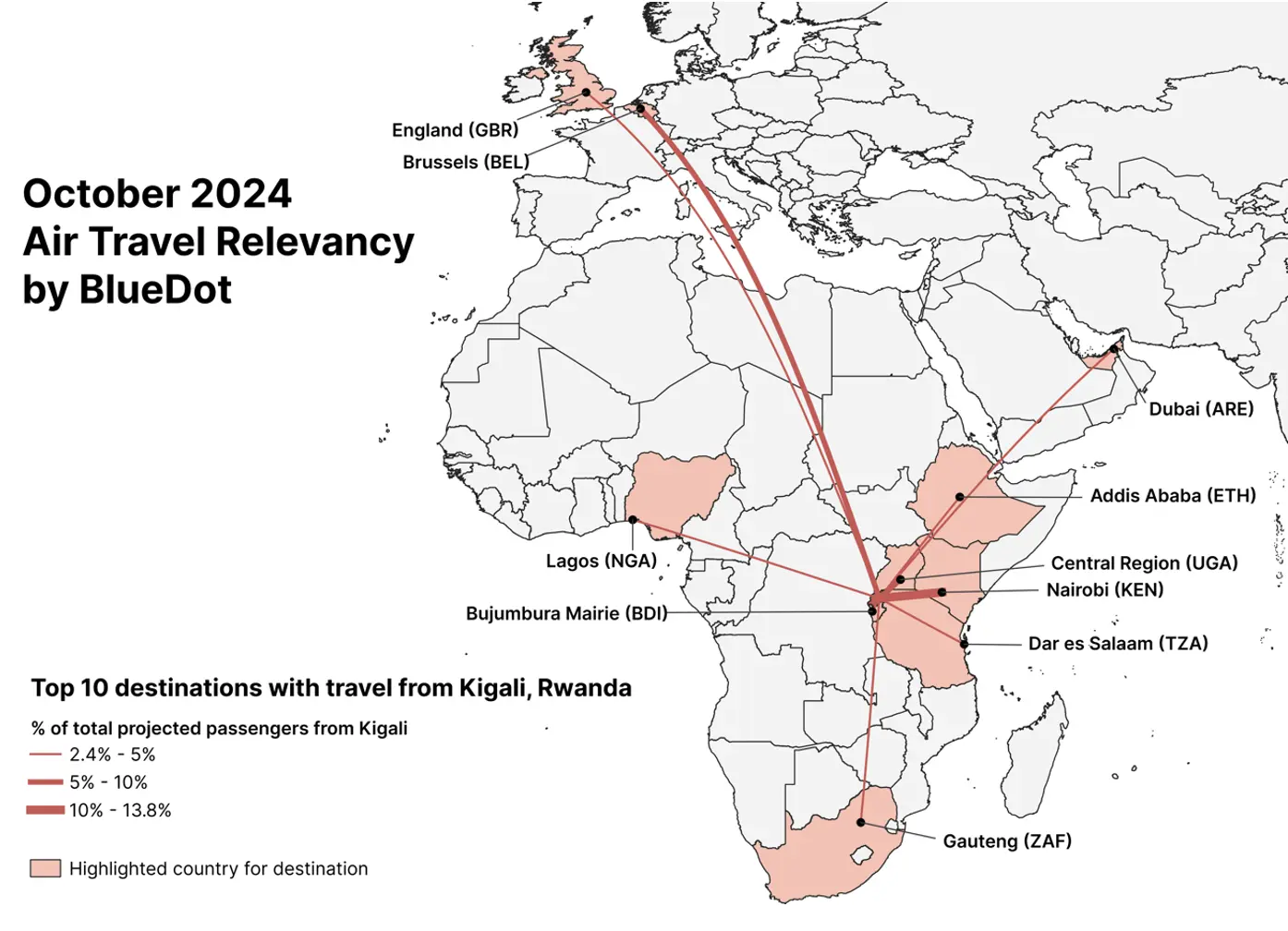
A ground spread model developed by BlueDot shows that Rwanda’s Eastern province has a 70% likelihood of receiving ground travelers from the capital region, followed by a 12% likelihood for the Southern province. Internationally, the country most likely to receive ground travelers from Kigali is the Democratic Republic of Congo, with a 2% likelihood for both its North Kivu and South Kivu provinces.
Meanwhile, BlueDot’s air travel relevancy assessment for flights from Kigali region shows that the highest proportion of travelers (13%) are projected to go to Nairobi, Kenya, while 6% are projected to go to Brussels, Belgium. Given overall air travel volumes and the projected size of the outbreak, the risk of spread due to air travel is considered low.
4 Top Takeaways
- Rwanda is experiencing its first ever Marburg outbreak. To date the country has identified 65 cases and 15 fatalities due to the virus, which causes haemorrhagic fever similar to Ebola.
- The country has responded swiftly and effectively. The country has administered more than 3,000 tests and has isolated dozens of close contacts of infected individuals. The Africa CDC reports that the outbreak is under control.
- The outbreak is the site of a Phase 2 vaccine trial. The total of 1,700 doses of a phase 2 Marburg virus vaccine in development have been provided to Rwandan health authorities, who have moved quickly to administer them.
- Marburg virus outbreaks are increasing in frequency. Once sporadic, the highly lethal virus has caused outbreaks on the African continent in each of the last three years.
Phase 2 open-label vaccine trial now underway
While Marburg virus outbreaks have historically been sporadic in nature, the increasing frequency of outbreaks in recent years have highlighted the need for prophylactic and antiviral measures specifically targeting the virus. While there are currently no licensed vaccines or treatments for Marburg virus disease, one candidate manufactured by Sanofi demonstrated robust immune responses and no safety concerns in Phase 1 clinical trials and nonclinical studies earlier this year.
It’s this investigatory vaccine that the Sabin Vaccine Institute has provided to the Rwanda Biomedical Centre as part of a phase 2 clinical trial agreement to administer the vaccine to high-risk adults. Given the high risk for health care workers, the vaccine is being rolled out to this population at six clinical trial sites. The initial agreement called for 700 single-dose vaccines to be administered; this past week the trial was expanded for an additional 1,000 doses.
This particular phase 2 study is open-label, meaning that its clinical characteristics have been amended to be deployed during unexpected events — in this case, the live outbreak. Although drug development typically involves strict constraints, evaluating the efficacy and safety of vaccines for rare viruses can only occur in outbreak environments. “This kind of open-label approach has been used in the past with Ebola virus outbreaks,” says Dr. Torres Portillo. “Given the strong results of the phase 1 trials and the severity of the illness caused by Marburg virus, it’s an appropriate step.” A proven, licensed vaccine would have a significant impact on the course of future outbreaks, providing public health authorities with a crucial defense against the spread of this lethal virus.
On our radar
Other developments we’re tracking at BlueDot
- Dengue in California: A locally-acquired case of dengue was recently identified in San Diego County, which until now had only recorded 49 travel-related dengue cases. Los Angeles has also recorded five locally-acquired cases. Southern California is now within the range of suitable climate conditions to support the Aedes mosquito, the vector that carries the dengue virus, meaning local outbreaks of the virus may become more regular occurrences.
- Oropouche in body fluids: Recent research identified viable Oropouche virus (which Outbreak Insider covered back in June of this year) in the semen of an infected individual, which raises the likelihood that the disease can also be transmitted sexually and could cause complications during pregnancy.
To keep updated on infectious disease outbreaks around the globe, sign up here to receive every edition of BlueDot Outbreak Insider