Since the beginning of 2020, a vaccine for COVID-19 has been held up as a silver bullet that will stop the pandemic. According to BlueDot’s latest COVID Focus Report, this is unlikely to be the case. That’s because the primary target endpoints for vaccine efficacy in the 11 vaccine candidates currently undergoing Phase 3 trials are to prove a lower risk of the COVID-19 disease manifestation from the presence of the virus, but not necessarily to prove prevention of infection.
BlueDot regularly produces COVID Focus Report for clients around the world. Here are highlights from this one:
Executive Summary
- Eleven COVID-19 vaccine candidates are currently being studied in Phase 3 or Phase 2/3 trials. All vaccine candidates are being tested in adult populations and three are also being tested in pediatric populations.
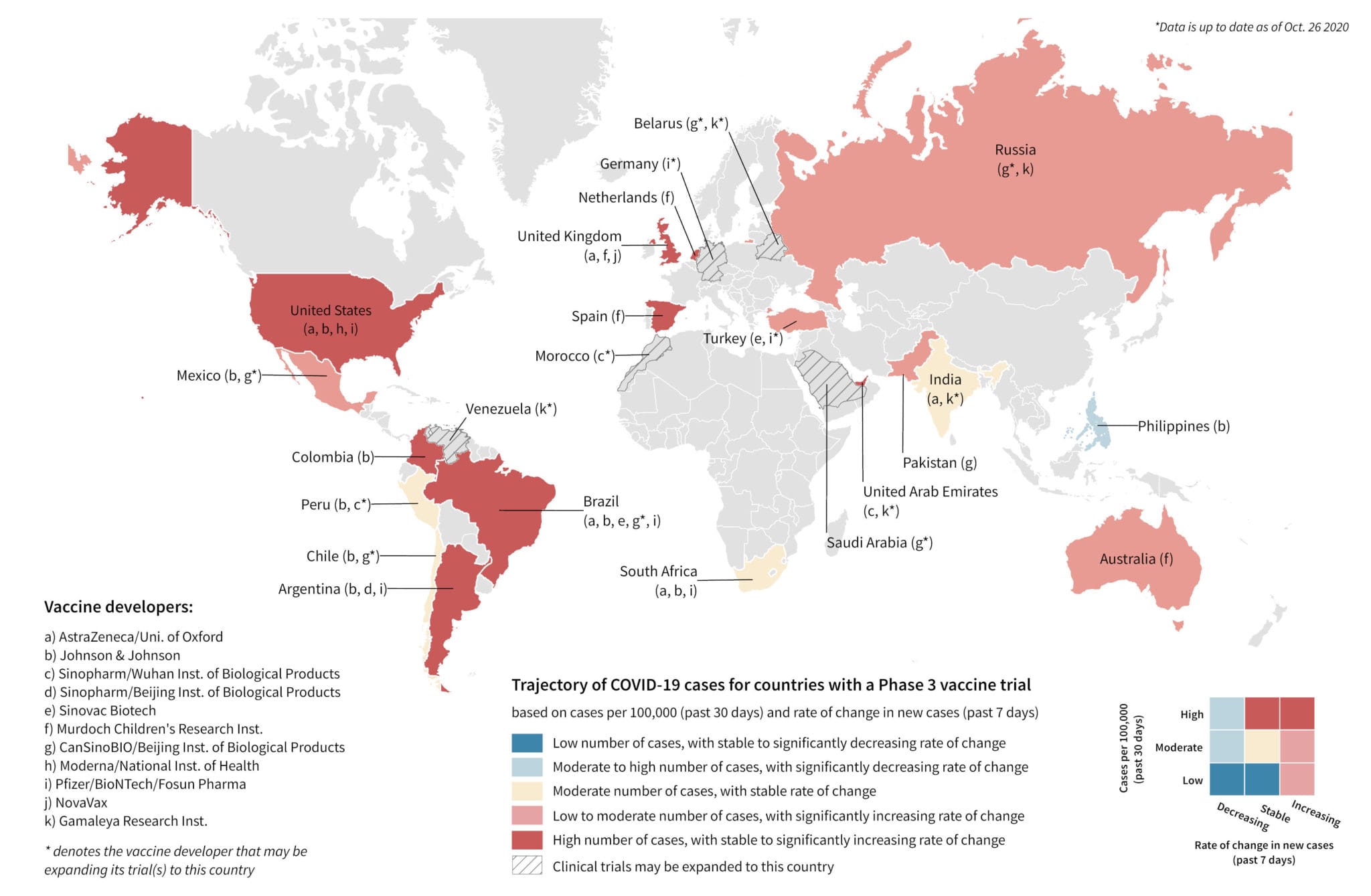
- These studies are currently being conducted across 18 countries, with the United States and Brazil being the most popular locations. Nearly all study locations included in the trial have experienced a wave of growth and/or are currently experiencing a growth in COVID-19 cases at the country level since the trials began. Study sites with high transmission rates of SARS-CoV-2 are more likely to reach their pre-specified endpoints sooner in order to establish the efficacy of a vaccine candidate.
- These studies are anticipated to be fully completed sometime between 2021 and 2023, with the earliest anticipated completion date of May 2021 for two vaccine candidates. Completion dates may change based on the progress in recruiting participants, achieving event-driven endpoints, and potential pauses for investigations of reported adverse events, which are a routine procedure in clinical trials.
- On October 25 2020, Dr. Anthony Fauci (U.S) speculated that the vaccination of a “substantial proportion of the population” will likely not occur until “the second or third quarter of [2021]”.
- Across all countries, the United States government has announced the most public spending on COVID-19 vaccine development in total, followed by Chile.
- A recent global online survey of adults across 19 countries found that 71.5% of respondents “somewhat agree” or “completely agree” with the statement: “If a COVID-19 vaccine is proven safe and effective and is available to me, I will take it”. However, responses vary across different countries.
What does this mean?
- As many of these trials are event-driven (i.e., evaluation of vaccine efficacy is conducted when the number of study participants who are diagnosed with COVID-19 reaches a pre-determined threshold), the growing country-level transmission of SARS-CoV2 may accelerate the Phase 3 vaccine trials’ anticipated timeline.
- It is likely that regulatory agencies may begin preliminary assessments of a vaccine candidate’s efficacy and safety using interim data before the trial is fully complete. However, early approval of a potential COVID-19 vaccine may be limited to specific populations, such as healthcare providers. Full approval of vaccine use in a broader population in most countries will likely await full completion of safety data.
- Positive results from a study on vaccine candidates might not necessarily translate to its availability globally. Vaccine developers must receive approval from each country’s regulatory agency (i.e. the Food and Drug Administration for the U.S., Health Canada for Canada, European Medicines Agency for the E.U) prior to its widespread use in that location. Some U.S. states (e.g. California, Washington, Oregon, and New York) have announced that they will also independently assess the efficacy and safety of vaccine candidates from the study results prior to approval.
- Despite the accelerated development of COVID-19 vaccine candidates, various factors can influence the access to an approved vaccine and its impact in preventing COVID-19 among populations. These include:
- the scale-up and cost of manufacturing and supply chain management for widescale distribution,
- the real-world effectiveness and safety of vaccines (which may differ from results under ideal conditions indicated by Phase 3 trials),
- the logistic considerations needed to store (i.e., some may require specific refrigeration prior to administration) and administer vaccines (i.e., number of doses required, prioritization of eligible populations), and
- the willingness of populations to receive a COVID-19 vaccine.
- The availability of a vaccine may not be a silver bullet to rapidly ending the spread of SARS-CoV-2 as the primary target endpoints for vaccine efficacy in Phase 3 trials are to prove a lower risk of the COVID-19 disease manifestation from the presence of the virus, but not necessarily to prove prevention of infection.